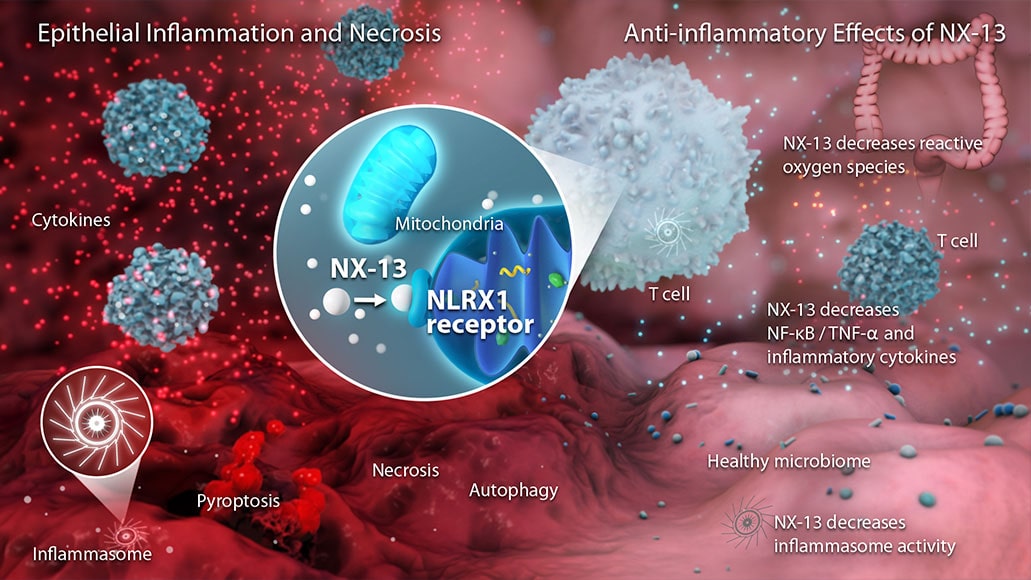
Landos has identified novel pathways including NLRX1 and PLXDC2. The NLRX1 pathway works to decrease reactive oxygen species and downregulate effector immune responses. The PLXDC2 pathway leads to the production of IL-10 and prevention of oxidative stress when activated.
Home Illustration
NX-13 is an oral NLRX1 agonist for the treatment of ulcerative colitis and Crohn’s disease. NX-13’s bimodal mechanism of action aims to decrease reactive oxygen species and oxidative stress, while decreasing pro-inflammatory signals.